Continued operational progress and strengthened financial position provides capital for key value inflection points and continued investment in leading technology platform
- Full data expected in Q1 2023 for BEN-2293 Phase IIa clinical study; a potential treatment for atopic dermatitis and a topical, best-in-class PanTrk inhibitor
- Collaboration extended with AstraZeneca to four disease areas, now with a total of three novel targets selected into their drug development portfolio
- Expect CTA by late 2022 for BEN-8744; an oral PDE10 inhibitor and first-in-class treatment for ulcerative colitis
- Continuous advancement of the BenevolentAI Platform™, enhancing prediction quality and optimising tools for the discovery of novel targets across multiple modalities
- Cash and cash equivalents of £165.3 million providing sufficient capital to Q4 2024 for key value inflection points without including any future capital from licensing or collaboration agreements
London, UK, 27 September 2022: BenevolentAI (Euronext Amsterdam: BAI), a leading, clinical-stage AI-enabled drug discovery and development company, announces its unaudited interim results for the six months ended 30 June 2022.
Joanna Shields, Chief Executive Officer of BenevolentAI, said: “We are pleased with our performance for the first six months of 2022 as we continue to build both internal and external validation of the Benevolent Platform™, our AI-enabled drug discovery platform. We progressed our in-house pipeline to 13 named platform-generated drug programmes and over 10 exploratory stage programmes and consistently delivered in our collaboration with AstraZeneca, who expanded the collaboration into two new disease areas and selected an additional target during the period. In May, we also gained further clinical validation of our approach after the FDA issued a full approval of baricitinib, the COVID-19 treatment first identified by BenevolentAI.”
“The capital raised from the business combination and listing in April this year puts us in a solid financial position to progress our development portfolio through near and medium-term key value inflection points and allows us to increase investment in our differentiated technology. As we look to the future, BenevolentAI’s scalable platform and industry-leading novel target identification capabilities have the potential to transform drug discovery by radically improving our understanding of disease biology and ultimately increasing the probability of clinical success.”
Operational highlights (including post-period)
- Continued development of in-house pipeline across several disease areas with all programmes generated by the Benevolent Platform™, validating its disease-agnostic capabilities.
Clinical programme
- BEN-2293 – topical best-in-class PanTrk inhibitor for atopic dermatitis (AD); data expected in Q1 2023 and on track to complete Phase IIa clinical study by end-2022
- Aim to deliver one to two CTA/IND drug candidates per year
Pre-clinical programmes
- BEN-8744 – oral peripherally-restricted PDE10 inhibitor and first-in-class treatment for ulcerative colitis (UC); CTA expected by late 2022 and a Phase I study anticipated to start in early 2023
- BEN-9160 – oral best-in-class asset for amyotrophic lateral sclerosis (ALS); IND enabling studies are expected to start Q1 2023 pending read-out of the in-vivo efficacy model
- BEN-28010 – oral best-in-class centrally penetrant asset for glioblastoma multiforme (GBM); nominated as a clinical candidate with preparation for IND enabling studies ongoing
Drug discovery and target identification (ID)
- Working to transition at least one further project into lead optimisation in Q4 2022, with two projects already advanced in H1 2022
- Initiated two new drug discovery programmes in H1 2022 with a further two-to-three anticipated in Q4 2022
- Going forward, intend to focus internal Target ID capabilities on three specific therapy areas: immunology, oncology and neurology
- Strategy enables the Company to build focused expertise to successfully develop assets and, in the future, potentially commercialise with or without a partner
Consistent delivery in commercial Target ID collaboration with AstraZeneca (AZ) providing ongoing Platform validation:
- Collaboration initiated in 2019 to support novel target identification utilising AI and machine learning for two indications: chronic kidney disease (CKD) and idiopathic pulmonary fibrosis (IPF) with one target selected and validated in-house by AZ for each indication in 2021
- A three-year collaboration extension announced in January 2022, adding two new disease areas: systemic lupus erythematosus (SLE) and heart failure (HF)
- Second novel target selected for IPF in May, taking the total number of novel targets selected to three overall
- Collaboration provided upfront license fees with the potential for future development milestones and sales-based royalty revenues on any successfully commercialised asset
Full FDA approval of COVID-19 treatment first identified by BenevolentAI providing clinical and regulatory validation
- In May 2022, the US Food and Drug Administration (FDA) granted full approval for baricitinib (approved for rheumatoid arthritis and marketed by Eli Lilly) to treat COVID-19 in hospitalised adults requiring supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO), clinically validating BenevolentAI’s approach
- BenevolentAI first identified baricitinib as a repurposed drug candidate in 2020 using the Benevolent Platform™. Baricitinib delivered a 38% reduction in mortality in hospitalised patients, rising to 46% for those on supplemental oxygen, in Eli Lilly’s COV-BARRIER trial
- As previously disclosed, Eli Lilly subsequently invested in BenevolentAI in 2020
Non-commercial collaborations
- Initiated phase two of AI research partnership with the Stanford University-based Helix Group in the first half of 2022 to discover more effective methods to extract knowledge from biological and clinical information, discovering and adjudicating contradictions in scientific literature
- Initiated AI research collaboration with the Drugs for Neglected Disease initiative (DNDi) in April to identify potential biological targets and therapies that could be repurposed for dengue to prevent disease progression. Targets have since been provided to the DNDi for experimental validation
Continuous enhancement of the Benevolent Platform™
- Continued growth of data within Knowledge Graph primarily due to an increase in patient-level data (omics) and enhanced natural language processing (NLP) recall (46% of which is proprietary to BenevolentAI)
- Evolution of data representation and introduction of large language models (LLMs) improve quality of reasoning on scientific literature for better understanding of human disease aiming for higher quality target predictions
- Extensions made to the existing Benevolent PlatformTM to optimise the discovery of novel targets best prosecuted by alternative modalities (e.g., monoclonal antibodies). Expected to lead to a biologic entrant into the pipeline, further diversifying the portfolio
Corporate highlights
- Strengthened Board of Directors and Leadership
- Promoted Dr. Daniel Neil to Chief Technology Officer, and appointed Nicholas Keher as Chief Financial Officer and Dr. Nicola Richmond, GSK veteran, as Vice President of Artificial Intelligence
- Dr. Olivier Brandicourt, former CEO of Sanofi, Jean Raby, former CEO of Natixis Investment Managers, and Dr. Susan Liautaud, Stanford University Ethics Expert, appointed as Non-Executive Directors
- Kenneth Mulvany, Founder and Non-Executive Director, stepped down from the Board and Michael Brennan, Co-Founder and Non-Executive Director, will step down on 30 September 2022
- Continued to expand expertise across the business, including in drug discovery and development, with a focus on enhanced clinical capabilities
Financial highlights
- Revenue of £4.8 million (H1 2021: £1.7 million) driven by extended AstraZeneca collaboration
- Drug Discovery R&D expenditure of £19.3 million) (H1 2021: £13.0 million) reflecting continued investment in pipeline and Phase I/II activities on BEN-2293
- Operating loss of £55.3 million (H1 2021: £46.5 million) in line with internal expectations
- £177.9 million gross proceeds from the successful business combination and listing on Euronext Amsterdam completed April 2022
Analyst and Investor briefing
BenevolentAI's executive leadership team will be hosting a briefing for analysts and investors at 14:00 BST / 09:00 EST on 27 September 2022 at the offices of FTI Consulting (200 Aldersgate, Aldersgate Street, London, EC1A 4HD, United Kingdom). The presentation will also be accessible via webcast with a recording made available on the Company's website shortly afterwards. To register your interest in attending either in person or virtually, please contact FTI Consulting at BenevolentAI@fticonsulting.com or +44 (0) 20 3727 1000.
At the event, BenevolentAI's management team will lead sessions about the Company's approach and technology and its positioning within the wider AI drug discovery market, as well as talking about the Company's pipeline and business model. The event will also include an overview of BenevolentAI's interim results for the period ended 30 June 2022. Presentations will be given by BenevolentAI's executive leadership team in addition to Professor Tom MacDonald of Immunology at Barts and the London School of Medicine and Dentistry, Queen Mary, University of London.
No additional, material new information will be disclosed during the analyst and investor briefing.
Enquiries:
BenevolentAI:
Nick Keher, Chief Financial Officer
T: +44(0) 203 781 9360
Media:
Rajin Kang - VP Communications
T: +44(0) 203 781 9360
Investors:
Fleur Wood – VP Investor Relations
T: +44(0) 203 781 9360
FTI Consulting:
Ben Atwell/Simon Conway/Victoria Foster Mitchell
T: +44 203 727 1000
BenevolentAI@fticonsulting.com
About BenevolentAI
BenevolentAI (AMS: BAI) is a leading, clinical-stage AI-enabled drug discovery and development company listed on the Euronext Amsterdam stock exchange. Through the combined capabilities of its AI platform, scientific expertise, and wet-lab facilities, BenevolentAI is well-positioned to deliver novel drug candidates with a higher probability of clinical success than those developed using traditional methods. The Benevolent Platform™ powers a growing in-house pipeline of 13 named drug programmes and over 10 exploratory programmes, and it maintains successful collaborations with AstraZeneca, as well as leading research and charitable institutions. BenevolentAI is headquartered in London, with a research facility in Cambridge (UK) and a further office in New York.
Forward-looking Statements
This release may contain forward-looking statements. Forward-looking statements are statements that are not historical facts and may be identified by words such as "plans", "targets", "aims", "believes", "expects", "anticipates", "intends", "estimates", "will", "may", "should" and similar expressions. Forward-looking statements include statements regarding objectives, goals, strategies, outlook and growth prospects; future plans, events or performance and potential for future growth; economic outlook and industry trends; developments in BenevolentAI’s markets; the impact of regulatory initiatives; and/or the strength of BenevolentAI’s competitors. These forward-looking statements reflect, at the time made, BenevolentAI’s beliefs, intentions and current targets/aims. Forward-looking statements involve risks and uncertainties because they relate to events and depend on circumstances that may or may not occur in the future. The forward-looking statements in this release are based upon various assumptions based on, without limitation, management's examination of historical operating trends, data contained in BenevolentAI’s records, and third-party data. Although BenevolentAI believes these assumptions were reasonable when made, these assumptions are inherently subject to significant known and unknown risks, uncertainties, contingencies and other important factors which are difficult or impossible to predict and are beyond BenevolentAI’s control. Forward-looking statements are not guarantees of future performance, and such risks, uncertainties, contingencies and other important factors could cause the actual outcomes and the results of operations, financial condition and liquidity of BenevolentAI or the industry to differ materially from those results expressed or implied by such forward-looking statements. The forward-looking statements speak only as of the date of this release. No representation or warranty is made that any of these forward-looking statements or forecasts will come to pass or that any forecast result will be achieved.
OPERATING REVIEW
We are pleased to present our first set of financial results following our business combination and listing on Euronext Amsterdam in April 2022, including an update on the operational progress achieved by BenevolentAI during the Period.
BenevolentAI is a leading, clinical-stage AI-enabled drug discovery company with a track record of producing scientifically-validated drug discoveries by delivering novel drug candidates with a higher probability of clinical success than those developed using traditional industry methods.
We combine advanced AI and machine learning (ML) with cutting-edge science to decipher complex disease biology. Our differentiated approach through the Benevolent Platform™ empowers creativity and innovation in drug discovery and development. This approach has generated a growing in-house pipeline of 13 named drug programmes and over 10 exploratory programmes while supporting successful commercial collaborations with AstraZeneca and leading global research and charitable institutions.
The Benevolent Platform™ is a validated AI-enabled hypothesis-driven platform that powers high-confidence drug discovery. It is disease and modality-agnostic and capable of rapidly generating novel targets at scale. Our corporate strategy to date has been to leverage the Benevolent Platform™ to identify novel drug targets across a range of different therapeutic areas with the aim to reduce time and cost and improve the probability of clinical success compared to industry standards.
Following a strategic review completed during the period by the Board, the Company will now look to focus its in-house pipeline on three specific therapy areas: immunology, oncology and neurology. This focused approach to our strategy will allow us to take forward assets where we have the right capabilities to successfully develop and, in the future, potentially commercialise with or without a partner. Subsequently, at an appropriate time, we will look to out-licence assets in therapeutic areas requiring larger and more complex clinical trials or in areas where we do not have the optimal internal capabilities to develop further.
Over the period, we have invested in expanding our expertise by increasing headcount to over 350. Anticipated hiring will be weighted towards drug discovery and development, with a particular focus on enhanced clinical capability.
The following highlights the progress across our lead in-house clinical and preclinical pipeline assets and the continuous enhancement of the Benevolent Platform™.
BEN-2293 - topical best-in-class PanTrk inhibitor in development to relieve inflammation and rapidly resolve itch in patients with atopic dermatitis (AD): Phase IIa data expected in 2023
BEN-2293 is a drug candidate being explored as a treatment for mild and moderate atopic dermatitis, with the potential to be explored within severe atopic dermatitis. The molecule is an inhibitor of three tropomyosin receptor kinases (“Trk”), TrkA, TrkB and TrkC.
The Company is currently progressing a Phase Ib/IIa double-blind, placebo-controlled, first-in-human, two-part clinical study to investigate the safety, tolerability, PK and efficacy of repeat topical dosing of BEN2293 in 90 patients with mild to moderate AD. The Phase Ib portion of the study in 32 patients was successfully completed in December 2021. The Phase IIa portion in 90 patients is set to complete by end-2022 with data expected in Q1 2023. Efficacy measures incorporated in the study included changes to the eczema area severity index (EASI), affected body surface area (BSA), a validated investigator global assessment (vIGA-AD), a NRS itch scale, time to itch reduction and quality of life questionnaires.
The Benevolent Platform™ identified the role of the Trk receptors as mediators of both itch and inflammation in atopic dermatitis. Applying expertise in molecular design, we were able to design potent and selective inhibitors specifically of the three Trk receptors with high selectivity. We believe that BEN-2293 has the potential to demonstrate efficacy against both itch and inflammation with fewer side effects than steroid creams and various inhibitor treatments that are currently the dominant forms of treatment for this chronic condition.
If BEN-2293 successfully completes this clinical trial, our intention is to partner this asset with a pharmaceutical company that has a focus in dermatology for continued clinical development and, if approved, commercialisation. The addressable market for atopic dermatitis is forecast to exceed $14bn in the seven major markets by 2028.
BenevolentAI has filed for formulation and composition of matter patents covering BEN-2293.
BEN-8744 is an orally administered, peripherally-restricted, PDE10 inhibitor under development as a first-in-class treatment for refractory ulcerative colitis (UC)
IND-enabling studies are ongoing, and a Clinical Trial Application (CTA) is scheduled to be filed by late 2022, with the Phase I trial anticipated to start in early 2023.
The Benevolent Platform™ identified PDE10 as an entirely novel target for the treatment of UC – there was no previously known link between PDE10 and UC or other related inflammatory conditions. The target was experimentally validated in ex vivo UC colon samples from patients with active UC, allowing BenevolentAI to demonstrate efficacious target enzyme inhibition. Accordingly, we will look to demonstrate that BEN-8744 is effective in the treatment of moderate-to-severe cases of UC and with fewer side effects than the anti-TNF and JAK inhibitors that are currently the dominant form of treatment for this disease.
Our aim is to progress BEN-8744 through clinical trials and retain commercial rights to this asset in-house. The combination of high and growing disease prevalence, improved diagnosis, high treatments rates and the existence of numerous drugs in the development pipeline, together with the need for better treatments, is driving a market that is forecast to exceed US$7.8 billion by 2026.
BenevolentAI has filed for second medical use and composition of matters patents in respect of BEN-8744.
BEN-9160 is an orally administered, brain-penetrant asset we are developing as a best-in-class treatment for amyotrophic lateral sclerosis
BEN-9160 is a c-Abl inhibitor. Preclinical safety, drug metabolism and pharmacokinetics (“DMPK”) studies were completed in 2022. IND-enabling studies are expected to start Q1 2023, pending read out of an in-vivo efficacy model. Subject to positive data the asset will then be ready for Phase I studies in 2024.
The Benevolent Platform™ identified the c-Abl enzyme as a target for the treatment of ALS. C-Abl inhibitors had previously been studied primarily in the context of cancer treatments, but in vitro ALS models indicate that BEN-9160 also has the potential to be significantly neuroprotective, and we hope to demonstrate the drug candidate’s efficacy in delaying the progression of ALS in our planned clinical development programme.
BenevolentAI has filed for composition of matter patents covering BEN-9160.
BEN-28010 is an orally administered asset we are developing as a best-in-class treatment for glioblastoma multiforme
This asset was declared a clinical candidate, post period, in July 2022 with preparation for IND-enabling studies ongoing. Subject to positive data the asset will then be ready for Phase I studies in 2024.
BenevolentAI has filed a number of patents covering BEN-28010.
Earlier stage pipeline assets
Across the rest of the pipeline we continue to see good progress, with two assets progressing into lead optimisation in the period and a further two assets expected to enter lead optimisation in the second half of 2022. We have also started two new drug programs in the period, with a further two-to-four expected to enter our pipeline in the second half of 2022 and with all targets generated from the Benevolent Platform™.
Continuous enhancement of the Benevolent Platform™
The Benevolent Platform™ powers a growing pipeline of in-house assets and collaborations. The platform is an AI-enabled, hypothesis-driven drug discovery methodology that draws on a multi-modal biomedical knowledge base and enables scientists to explore underlying disease mechanisms and objectively identify potential novel drug targets.
The platform improves predictions by running repeatable, reproducible processes at scale and continuously learns from insights generated from experimental data generated from the Company’s wet-laboratory facilities and AI models. The impact this continuous learning loop has on our platform increases over time, enabling the company to further enhance target identification and accelerate end-to-end drug discovery.
The BenevolentAI Data Foundations sits at the core of our Platform and drug discovery programmes and is built on the foundations of all publicly relevant biomedical data mined from structured sources and language data, including scientific and patent literature, regulatory documents and primary human data, as well as third-party proprietary data and our in-house experimental data. The Knowledge Graph is constantly enriched to supply a comprehensive representation of biomedical data. Our exploratory tools and predictive models empower scientists to explore relationships in the graph between biological entities and disease networks.
We continue to grow and enrich our Data Foundations with new and generated insights primarily due to an increase in patient-level data (‘omics) and enhanced natural language processing (NLP) recall. This led to an increase of over 250% in relationship volume in the Knowledge Graph from June 2021 to August 2022 (46% of which is proprietary to BenevolentAI) albeit we expect this growth rate to moderate in future years. The Knowledge Graph continues to evolve, and a new version was launched during the period to enhance the recall of facts from scientific literature, supporting more nuance and specificity in our biological relationships.
Beyond our data representation, our AI tools for scientists and predictive algorithms have improved at pace. Extensions and enhancements to our existing suite of tools have been completed that allow for novel targets best prosecuted by alternative modalities for example, monoclonal antibodies. We have also introduced a large language model (LLM) approach for our tools for more sophisticated reasoning.
Business model
The Company’s flexible business model allows for different approaches to drug discovery and development, which can be tailored to suit each individual programme. It can out-license drug candidates at different stages of clinical development and, therefore, adjust the required level of funding over time. Furthermore, with a validated platform that is agnostic to therapeutic area and drug modality, we can select the opportunities with the most attractive value creation potential.
The Company will now seek to develop further and then partner and/or commercialise pipeline assets in the following three disease areas: immunology, oncology and neurology. In all other disease areas assets would be out licensed prior to entering the clinic.
In addition to our in-house pipeline, we will also target a small number of additional platform collaborations, similar to those with AstraZeneca. This would provide non-dilutive funding, further validate our platform and, importantly, allow us to leverage the data generated to enhance our Knowledge Graph to the benefit of our in-house pipeline.
We expect to generate revenue broadly from three streams:
- Out-licence revenue: For some drug programmes, we will choose to out-licence to partners, who will then assume responsibility for some or all of the remaining clinical development and commercialisation. At the point of out-licensing each drug candidate, we expect to receive an up-front payment and then to receive milestone payments upon successfully completing various clinical, regulatory and/or sales milestones by the licensor. In addition, we would expect to receive royalty payments on the net sales of the out-licensed drugs.
- Platform collaboration revenue: We may receive upfront payments, research funding, milestones and royalties from platform collaborations. Platform collaborations are where we work with a partner to identify new drug targets using the Benevolent Platform™. This includes the current collaboration with AstraZeneca, which began in 2019 and has recently been extended until 2025. The AstraZeneca collaboration has been a primary driver of revenue over the past three years.
- Product sales from self-commercialised assets: We intend to commercialise certain drugs discovered using the Benevolent Platform™. Our first product launch is targeted for the second half of this decade or shortly thereafter, and we plan to build all the necessary infrastructure to successfully launch and commercialise our drugs globally.
Outlook
Our listing in April 2022 has strengthened our financial position and will enable us to enhance our leadership in the AI drug discovery sector. We believe that our scalable Benevolent Platform™ will transform understanding of disease biology and help us uncover novel treatment approaches faster, and with a higher probability of clinical success.
Due to the flexibility and disease agnostic capability of the Benevolent Platform™, we aim to monetise our technology in three ways, as outlined below, to increase shareholder returns over the coming periods.
- Our primary focus will be to independently pursue the clinical development of certain in-house pipeline assets in a number of selected core therapeutic areas, identified as immunology, oncology and neurology, before either commercialising these in-house or with partners. Our lead asset for this approach is BEN-8744 for ulcerative colitis, which will enter Phase 1 first in human trials in 2023.
- We will out-license pipeline assets that do not fit within these new target indications at selective times to maximise value. Our lead asset for this approach is BEN-2293, which we will aim to out-license in 2023 post Phase 2 data.
- Lastly, we will endeavour to enter additional strategic collaborations to leverage our disease agnostic capabilities into therapeutic indications outside our focus areas.
By the end of 2022, excluding early discovery programmes, BenevolentAI expects to have initiated 17 named drug programmes and, in 2023, expects to add between four and six named
drug programmes. In addition, BenevolentAI will aim to progress one to two CTA/IND-stage drug candidates every year, not including assets taken forward by collaboration partners. This target number could increase if funding allows due to the scalability of our platform.
BenevolentAI is well-funded with several value inflection points both in the near and medium term, providing a solid platform to fulfil our mission of delivering life-changing medicines to patients.
Joanna Shields
Chief Executive Officer
27 September 2022
FINANCIAL REVIEW
Business combination
On 22 April 2022, Odyssey SPAC and BenevolentAI Limited (the former parent company of the BAI Group before the Business Combination (“Transaction”)), entered into a business combination agreement by way of contribution of all shares in BenevolentAI Limited into Odyssey SPAC in exchange for the issuance of new ordinary shares. The Transaction was completed on 22 April 2022 and resulted in changing the name of the Group’s new holding company from Odyssey Acquisition S.A. to BenevolentAI (the “Group”).
As part of the overall Transaction an equity fundraise via a private investment in public equity (“PIPE”) and backstop arrangement was concluded, where the Group received at closing a cash flow of £177.9 million, with transaction costs reflected in the P & L for the period of £79.3 million. As part of the Transaction related costs the Group incurred a non-cash, non-recurring listing service expense of £65.4 million, professional and bank fees of £11.3 million and share-based payment expenses of £2.6 million, which have been removed from the reported normalised operating loss to more accurately reflect the Group’s underlying performance for the six months ended 30 June 2022. Other costs related to the Transaction are reflected in the opening reserves of the merged Odyssey SPAC entity (brought into the Group as at 22 April 2022, retranslated from EUR to GBP using at a 0.8302 exchange rate), or were expensed as incurred in the 2021 year end under each corresponding entity.
Through the Transaction, warrant liabilities were also acquired by the Group. These had been previously issued by Odyssey SPAC, and recognised as liabilities, with that treatment continuing through the reported period due to precedent accounting requiring liability treatment where a warrant is denominated in a currency other than that of the functional currency. The warrants were revalued at closing of the transaction (£18.1 million) and are revalued at each reporting period end, with the fair value as at 30 June 2022 of £12.0 million, with £6.1 million recognised as finance income.
Revenues
The Groups’ revenues have increased by £3.1 million to £4.8 million (H1 2021: £1.7 million) with the majority reflecting a second AI-enabled drug discovery collaboration with AstraZeneca starting in January 2022, combined with revenue from a one-year extension on the initial AstraZeneca collaboration that began in late 2021 and in H1 a milestone related to the second novel target for idiopathic pulmonary fibrosis.
Research and development (R&D) expenses
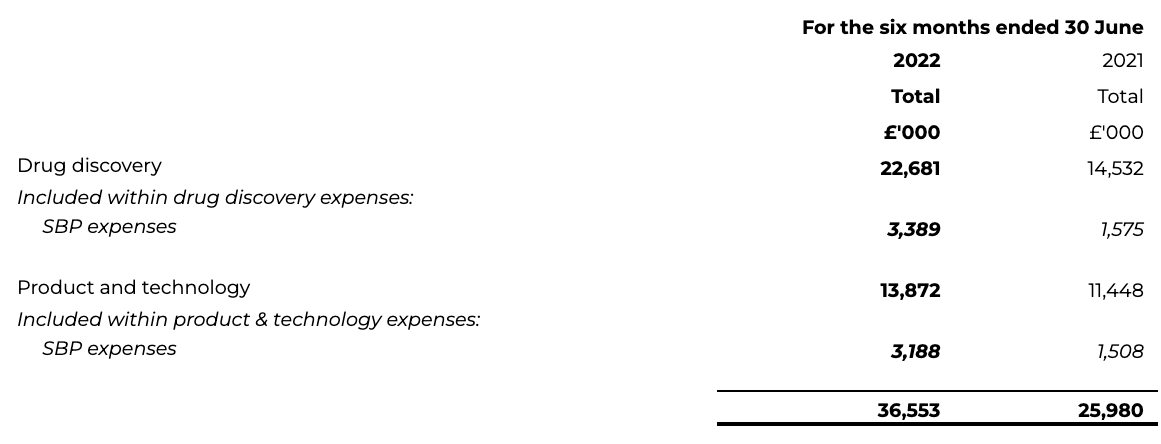
Normalised drug discovery spend, excluding share-based payments, for the first half of 2022 has increased to £19.3 million (H1 2021: £13.0 million), driven by the BenevolentAI pipeline advancing into later stages of development, in particular BEN-2293 and its progression through an adaptive Phase I/II clinical study.
Normalised product and technology spend for the first half of 2022 has increased to £10.7 million (H1 2021: £9.9 million), due to an increase in staff related costs to support the continued expansion of the Benevolent Platform™.
General and administrative costs
Normalised business operations spend, excluding share-based payments, for the first half of 2022 has increased to £8.1 million (H1 2021: £7.0 million), reflecting additional costs related to listing readiness. These costs are expected to stay at these levels given the enhanced level of compliance and reporting obligations.
Operating loss
Normalised operating loss for the first half of 2022 has increased to £55.3 million (H1 2021: £46.5 million).
Finance income
Finance income for the first half of 2022 has increased to £6.3 million (H1 2021: £10,000). This is predominantly driven by the fair value revaluation of the warrant liabilities acquired through the Transaction, reflecting a decrease in their value as at 30 June 2022 when compared to the Transaction date.
Taxation
Taxation income for the first half of 2022 has decreased to £3.9 million (H1 2021: £5.7 million). This is predominantly composed of tax credits arising from the UK’s small and medium-sized enterprises R&D tax relief regime, for which the increase on the claim between the two periods, driven by an increase in eligible R&D expenditure, is more than offset by the stamp duty tax payable of £3.7 million arising from the Transaction.
Loss per share
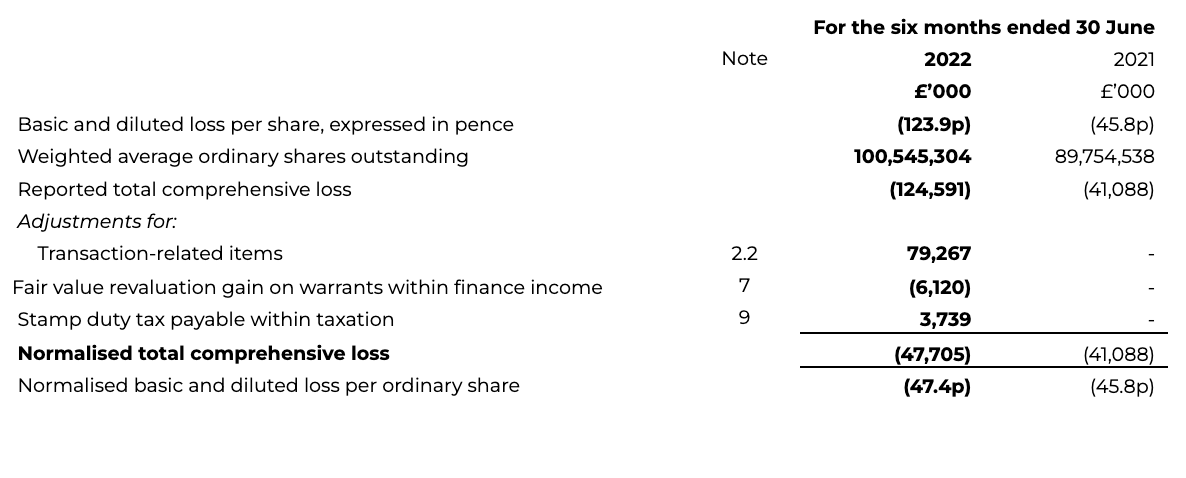
Normalised basic loss per share has increased to 47.4p for the first half of 2022 (H1 2021: 45.8p), with the weighted average number of shares in both periods adjusted to reflect the exchange ratio of the share for share exchange completed during the Transaction, such as to reflect the capital structure of the legal parent. The increase reflects the increase in normalised total loss.
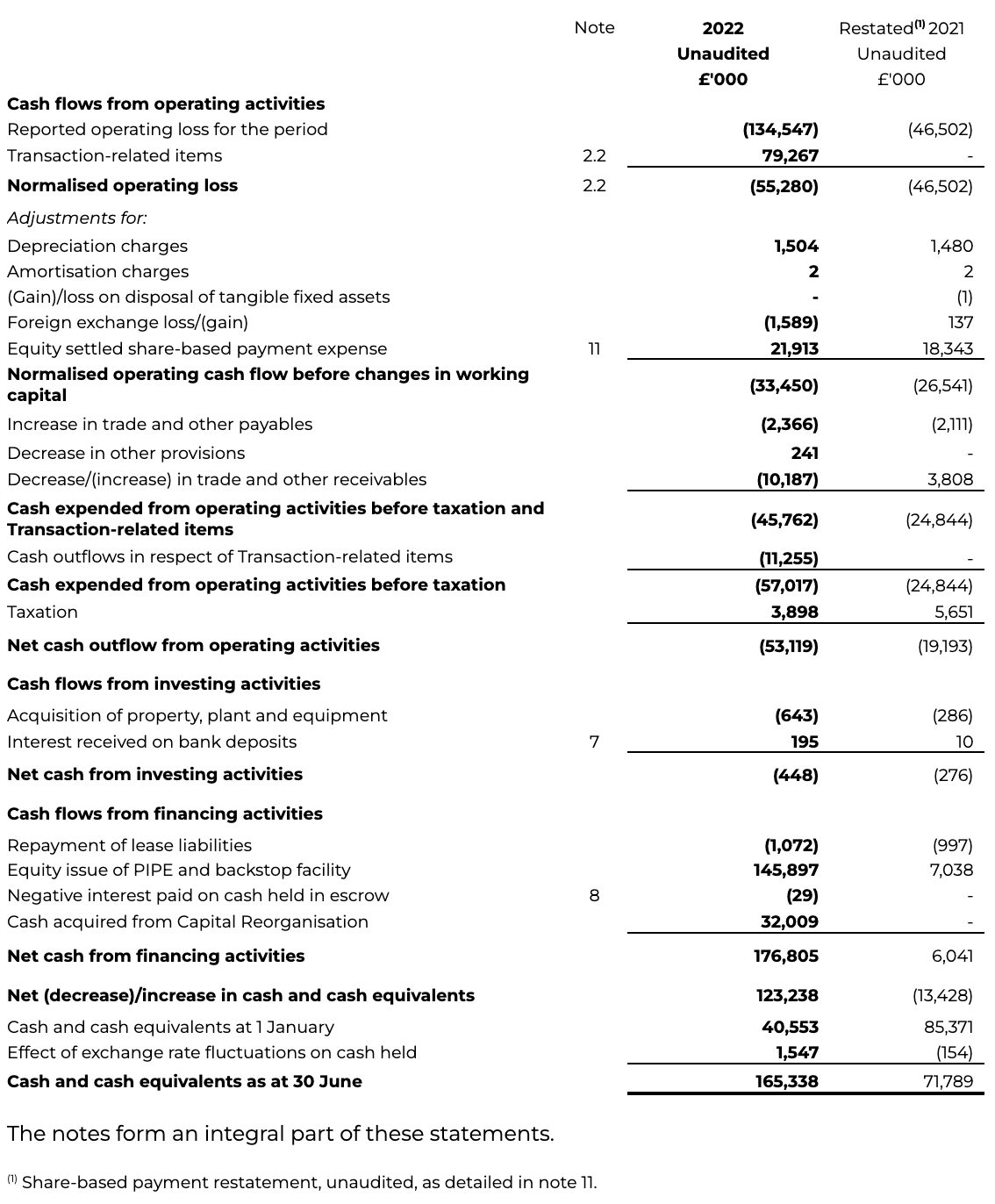
Current assets
Current assets as at 30 June 2022 have increased to £191.8 million (31 December 2021: £56.6 million), largely driven by a £124.8 million increase in cash.
Cash and cash equivalents
The cash position as at 30 June 2022 has materially strengthened with cash and cash equivalents of £165.3 million (31 December 2021: £40.6 million). This increase is driven by the PIPE, backstop proceeds, Odyssey Acquisition acquired cash, with gross proceeds of £177.9 million and AstraZeneca collaboration proceeds and off-set by the settlement of transaction expenses and ordinary course working capital expenditure.
Warrants
Warrants as at 30 June 2022 have increased to £12.0 million (31 December 2021: £nil), brought in from the Transaction and revalued at the reporting period end.
Other current liabilities
Other current liabilities as at 30 June 2022 have increased to £42.4 million (31 December 2021: £23.7 million), reflecting an increase in trade payables for Transaction-related expenses that were paid shortly after the Period ended, and an increase in deferred income under the AstraZeneca agreements.
Normalised Cash Flow
Cash expended from operating activities before taxation and Transaction-related items has increased to £45.8 million for the first half of 2022 (H1 2021: £24.8 million), primarily driven by the increase in normalised operating loss, as well as changes to working capital from outstanding receivables due on the R&D tax credits and AstraZeneca collaboration income receivables.
Nicholas Keher
Chief Financial Officer
27 September 2022
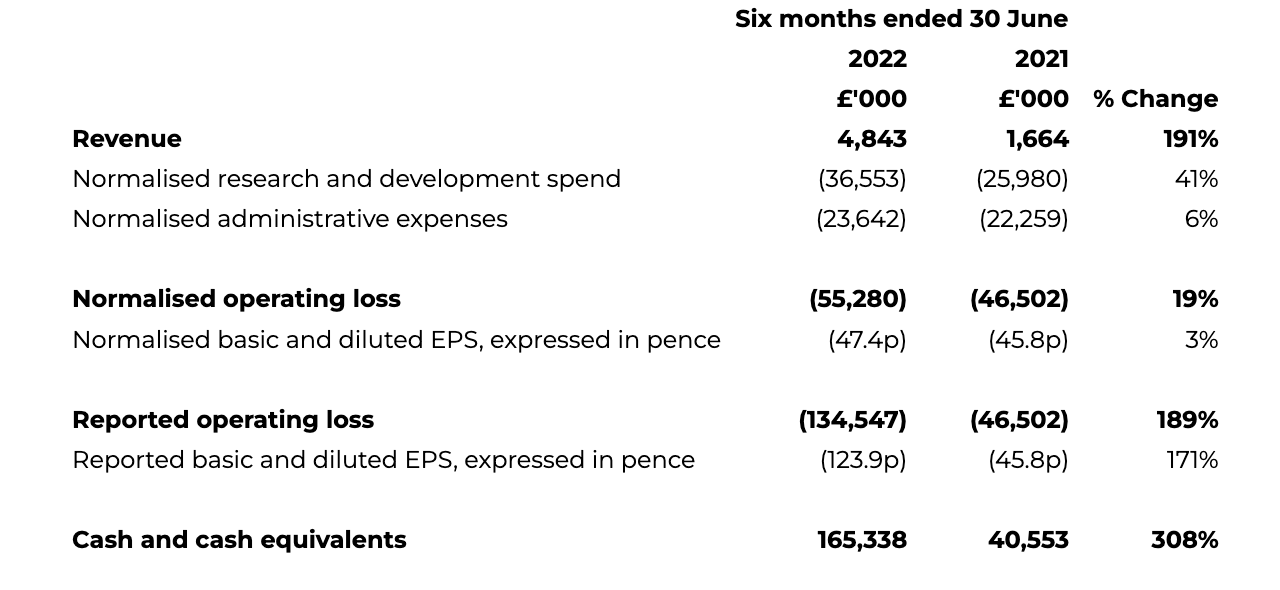
INTERIM CONDENSED CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME
for the six months ended 30 June
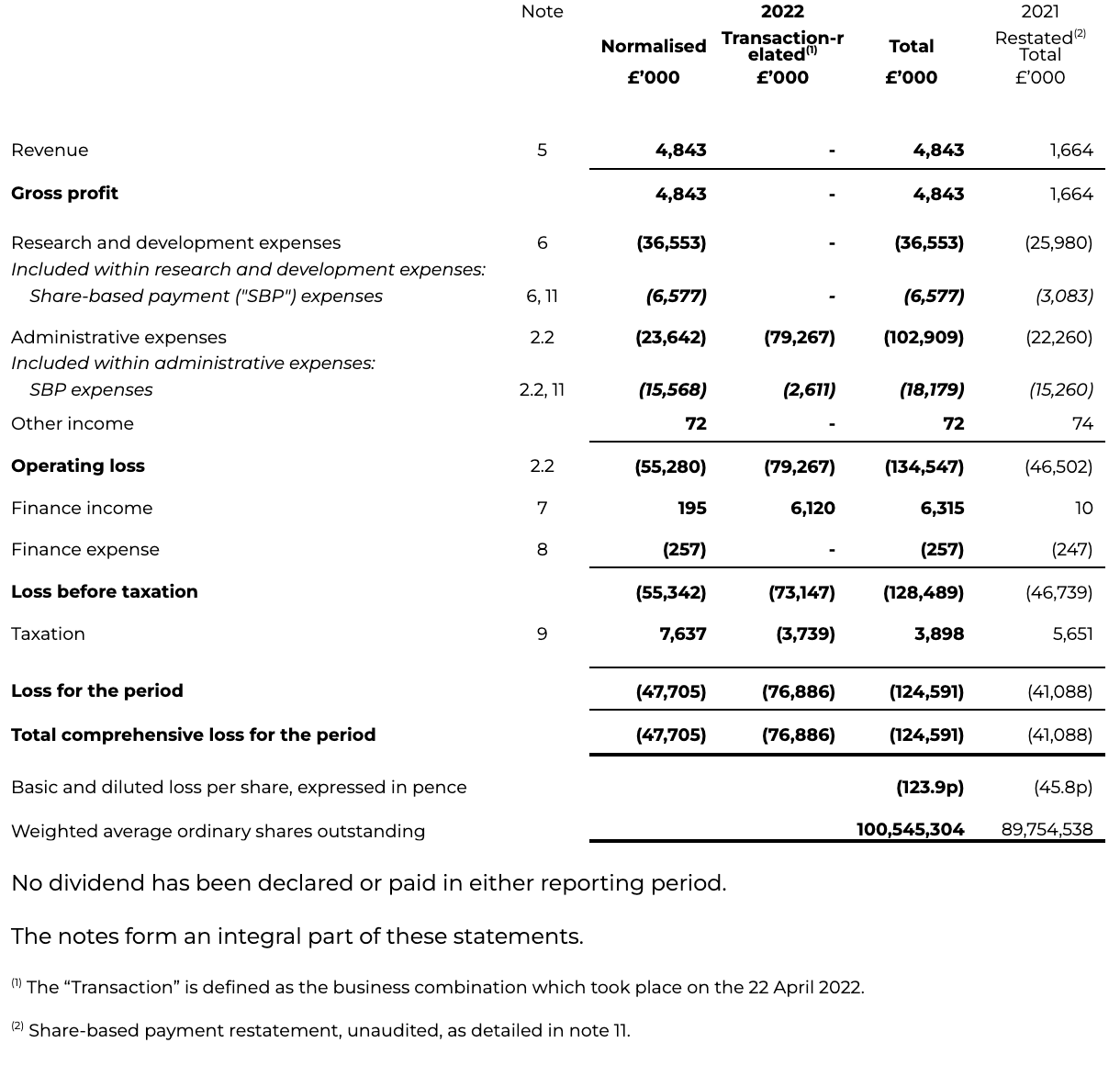
INTERIM CONDENSED CONSOLIDATED STATEMENT OF FINANCIAL POSITION
as at 30 June 2022
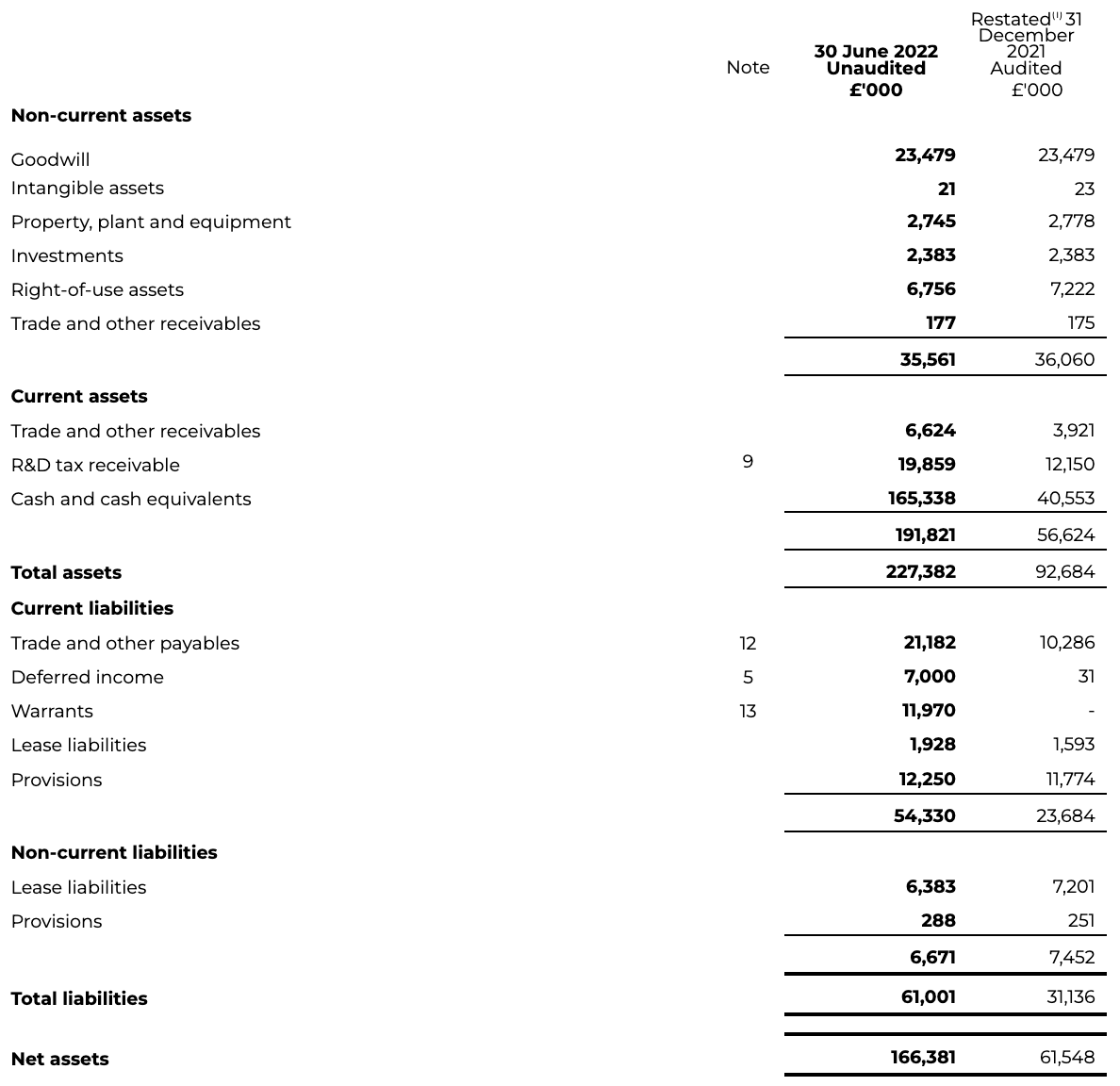

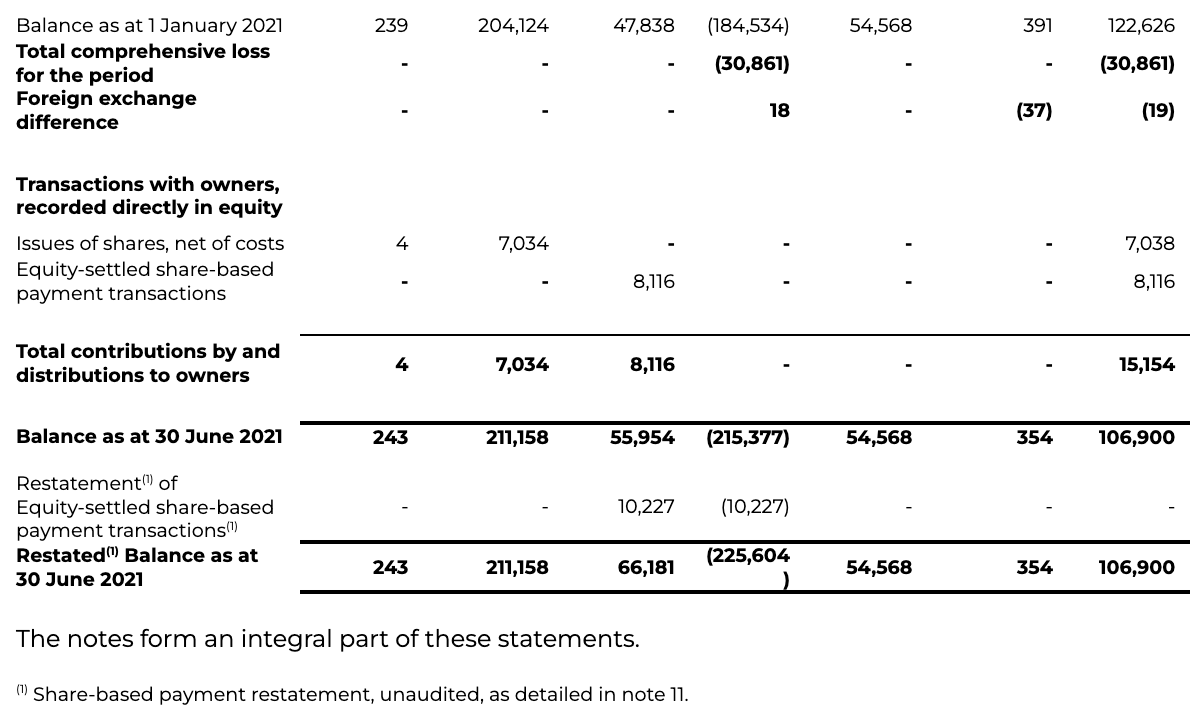
INTERIM CONDENSED CONSOLIDATED STATEMENT OF CHANGES IN EQUITY
for the six months ended 30 June 2022
INTERIM CONDENSED CONSOLIDATED STATEMENT OF CASH FLOWS
for the six months ended 30 June
NOTES TO THE FINANCIAL INFORMATION
1 Background to the Group
1. Corporate information
BenevolentAI (“the Company”) is a publicly listed company on the Euronext Amsterdam, with the ticker symbol BAI.
The Company is limited by shares, incorporated under the laws of Luxembourg under registered number B255412, having its registered office 9, rue de Bitbourg, L-273 Luxembourg, Grand Duchy of Luxembourg.
The principal activity of the Company and its subsidiaries (collectively, “the Group” or “BAI Group”) is that of creating and applying AI and machine learning to transform the way medicines are discovered and developed.
2. Group structure
BenevolentAI was originally known as Odyssey Acquisition S.A. (“Odyssey SPAC”), a Special Purpose Acquisition Company established for the purpose of acquiring a business with principal business operations in Europe or in another geographic area, that is based in the healthcare sector or the TMT (technology, media, telecom) sector or any other sectors. Odyssey SPAC was listed on the Euronext Amsterdam stock exchange on 6 July 2021.
On 22 April 2022 (“Closing date”), Odyssey SPAC and BenevolentAI Limited (the former parent company of the privately held UK group before the business combination (“Transaction”)), entered into a business combination agreement by way of contribution of all shares in BenevolentAI Limited into Odyssey SPAC in exchange for Odyssey SPAC issuing new ordinary shares. The Transaction was completed on 22 April 2022 and the name of the ultimate holding company was changed from Odyssey Acquisition S.A. to BenevolentAI, whose consolidated Group post-closing is referred to as BAI Group.
BAI Group is managed by its ultimate parent company BenevolentAI, with the following 5 trading subsidiaries operated under one segment.

2 Accounting policies
2.1 Basis of preparation
These unaudited interim condensed consolidated financial statements for the six months ended 30 June 2022 have been prepared in accordance with IAS 34 Interim Financial Reporting as adopted by the European Union. They have been prepared on a historical cost basis, except for financial instruments measured at fair value, and all amounts have been rounded to the nearest £ ‘000.
At 30 June 2022, net assets totaled £166.4m, with a cash balance of £165.3 million following the completion of the business combination. Management have reviewed the Group’s cash flow forecasts and believes it has sufficient cash for at least the next twelve months from the approval of these financial statements. The financial statements, therefore, continue to be prepared on the going concern basis.
The financial statements do not include all the information and disclosures required in the annual financial statements and should be read in conjunction with annual consolidated financial statements for the year ended 31 December 2021 for both Odyssey Acquisition S.A. and BenevolentAI Limited.
The Business Combination between BenevolentAI Limited and Odyssey SPAC is accounted for within the scope of IFRS 2 as a capital reorganisation since Odyssey SPAC did not meet the definition of a business in accordance with IFRS 3. Under this accounting method, Odyssey SPAC is treated as the acquired company for financial reporting purposes. The comparatives in the financial statements, therefore, represent the financial information of BenevolentAI Limited and its subsidiaries, both to 30 June 2021 and as at 31 December 2021. The activity and position of the acquired Odyssey SPAC is considered only from the Closing date onwards. That is, the statement of profit and loss contains only the post-acquisition performance of Odyssey SPAC, with the pre-acquisition losses being reflected through reserves. See note 4 for further details.
A number of new standards are effective for annual periods beginning on or after 1 January 2023 and earlier application is permitted; however, the Group has not early adopted the new or amended standards in preparing these consolidated Interim financial statements.
The consolidated interim financial statements of the Group for the six months ended 30 June 2022 were authorised for issue in accordance with a Board resolution on 27 September 2022.
2.2 Normalised operating loss
Normalised operating loss is defined as operating loss excluding expenses related to the business combination for the six months ended 30 June 2022. This is to show an underlying representation of operating losses and cash flows for the respective periods.
It is an alternative performance measure (‘APM’) that is not calculated in accordance with IFRS and therefore may not be directly comparable with other companies’ APMs, including those in the Group’s industry. APMs should be considered in addition to, and are not intended to substitute or supersede, IFRS measures.
This APM is in our view an important metric for a biotech company in the development stage. Removing the Transaction-related costs, given their isolated nature, enables users to better compare the Group’s normal operating performance between reporting periods.
The following table presents a reconciliation of normalised group operating loss, to the closest IFRS measures, for each of the periods indicated:

2.3 Change in functional currency of BenevolentAI
As of 22 April 2022, management reviewed the functional currency of BenevolentAI and the presentation currency of the Group. The change in functional currency, for standalone BenevolentAI was made to reflect that GBP has become the predominant operating currency for the Company representing a significant part of its cash flows and its operating environment, while the Group presentation currency remains in GBP consistent with the prior year.
The Group presents as comparative information the financial information of the former BenevolentAI Limited Group, which had GBP as its functional and presentation currency. Comparative information, therefore, has not been re-stated following this change to BenevolentAI’s functional currency.
3 Critical accounting estimates and judgements
The consolidated interim financial statements have been prepared in accordance with IFRS as adopted by the European Union and the application of the chosen accounting policies requires management to make judgements, estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses. The estimates and associated assumptions are based on historical experience and various other factors that are believed to be reasonable under the circumstances. Actual results may differ from these estimates.
The estimates and the underlying assumptions are subject to continuous review. The Group based its assumptions and estimates on parameters available when the financial statements were prepared. Existing circumstances and assumptions about future developments, however, may change due to market changes or circumstances arising that are beyond the control of the Group. Such changes are reflected in the assumptions when they occur.
In preparing these condensed interim financial statements, the significant judgements made by management in applying the Group’s accounting policies and the key sources of estimate uncertainty are:
Goodwill and Intangible Assets
The amount of goodwill and intangible assets initially recognised as a result of a business combination is dependent on the allocation of the purchase price to the fair value of the identifiable assets acquired and the liabilities assumed. The determination of the fair value of the assets and liabilities is based, to a considerable extent, on management’s judgement and based on industry benchmarks and information relevant to the specific assets in focus.
The carrying value of goodwill and intangibles requires the assessment of discounted future cashflows of the future economic value of the underlying assets. The assumptions and critical judgement required from management is inherently uncertain though based on recognised
industry methods of evaluating drug programme assets and companies undertaking drug discovery activities.
Management used industry practice in its method of evaluating the estimate of future revenue streams of drug programmes, associated costs together with market data for the therapeutic areas of interest, discount rates for cost of capital, risk factors including probabilities of progressing a candidate to commercialisation or earlier partnering of a programme and other relevant factors.
Management have further considered the positive progress identified to date from the acquisition of BenevolentAI Cambridge Limited with value anticipated to arise from already identified specific programmes in addition to the ongoing value creation from the underlying assets.
Share-based payments provision
The Group operates an unapproved Share Option Plan. All employees are offered options upon joining the Group. The fair value of share options granted is measured using the Black-Scholes model at each reporting date taking into account various assumptions detailed in note 12. The full charge of the vested options during the half year is recognised in the profit and loss.
Classification of Class A Warrants and B Warrants
As part of the Business Combination transaction, BAI Group took on warrants which had been initially issued by Odyssey SPAC prior to the Transaction, as part of financing Odyssey SPAC’s working capital and investment.
A derivative, other than a derivative that meets the definition of an equity instrument, is initially recognised as a financial asset or financial liability at its fair value on the date the derivative contract is entered into, and the related transaction costs are expensed. The fair values of the derivatives are remeasured at the end of each reporting period with changes in fair values recognised in net income or loss.
A derivative that will be settled by the Company delivering a fixed number of its own equity instruments in exchange for a fixed amount of cash in terms of its functional currency or another financial asset is classified and presented as an equity instrument, rather than a financial liability. The exercise price of the Company's share purchase warrants that are exercisable into common shares is denominated in EUR, however, the Company will receive a variable amount of cash in terms of its GBP functional currency upon exercise of the warrants due to movements in foreign exchange.
Accordingly, the Company's warrants are classified and presented as derivative financial liabilities and measured at fair value through profit or loss. The fair value of each warrant class is determined at each reporting date and exercise date and is based on quoted market prices, where available, or independently valued using Binomial Tree method and the Monte Carlo method. This is consistent with how fair value revaluations were determined under Odyssey SPAC.
4 Accounting impact of the Business Combination
Following the successful completion of the Business Combination with Odyssey SPAC on 22 April 2022, BenevolentAI became the new name of the holding company of the new BAI Group.
This Business Combination was achieved through the contribution of ordinary and preferred shares in BenevolentAI Limited in exchange for new ordinary shares in BenevolentAI (previously Odyssey SPAC), The result being that the new BAI Group became listed on the Euronext Amsterdam stock exchange.
As discussed in note 2.1, the Business Combination between BenevolentAI Limited and Odyssey SPAC was accounted for within the scope of IFRS 2 as a capital reorganisation, since Odyssey SPAC did not meet the definition of a business in accordance with IFRS 3. Accordingly, the Transaction was treated as the equivalent of BenevolentAI Limited issuing shares at the closing of the Business Combination for the net assets of Odyssey Acquisition S.A. as at the Closing date, accompanied by a recapitalisation. The excess of the fair value of consideration for Odyssey Acquisition S.A. over the fair value of its identifiable net assets acquired represents a compensation for the service of a stock exchange listing for its shares and expenses as incurred. This leads to a non-cash listing service expense of £65.4m that has been recognised in administrative expenses.
As at Closing date, the fair value of BenevolentAI Limited’s shares that were deemed to be issued to Odyssey SPAC amounted to £88.3m, based on the initial closing price of shares of Odyssey Acquisition S.A. according to the table below. In return, BenevolentAI received Odyssey SPAC’s listing service and its net liabilities, equal to £6.1m, which mainly consisted of remaining cash net of redemptions and liabilities related to the warrants, resulting in a total non-cash listing service expense of £65.4m to administrative expenses, excluding the £29.0m of Transaction-related expenses incurred by Odyssey SPAC.
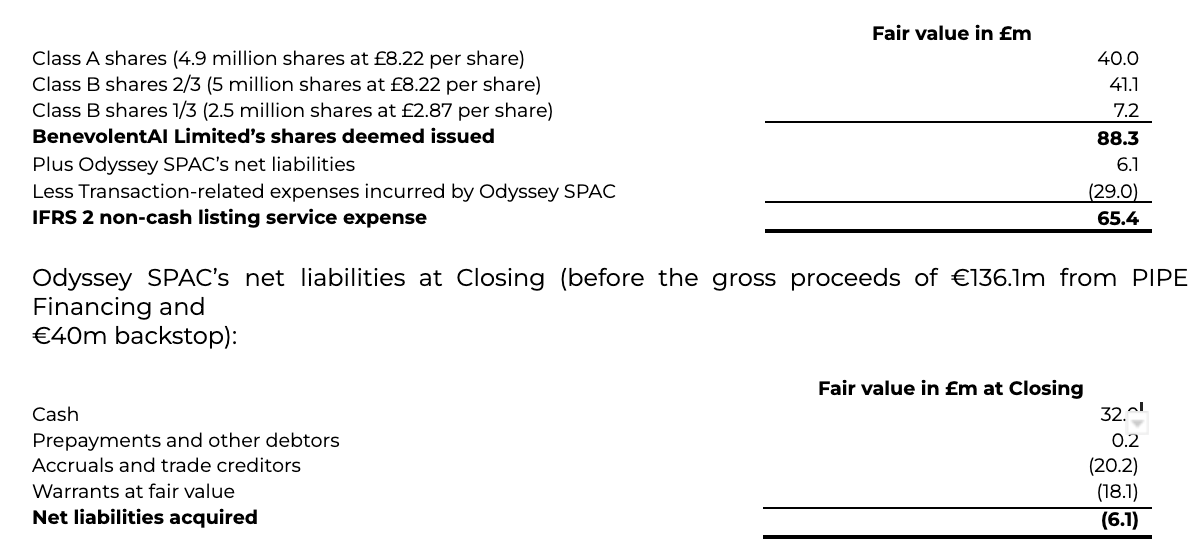
In conjunction with the Transaction, Odyssey SPAC entered into subscription agreements with investors (“PIPE Investors”) in a Private Investment in Public Equity transaction (the “PIPE Financing”) in the aggregate amount of €136.1m (£113.0m). In return for their investment, the PIPE Investors received a total of 13,613,394 additional Odyssey SPAC Class A shares. An equity back stop facility for €40m (£33.2m) resulted in a further issuance of 4,000,000 (per note 15) ordinary shares were also issued. This resulted in a total cash inflow of £145.9m (less £0.3m of professional fees) across the equity PIPE and Backstop.
Prior to closing, as consistent with the original public share offering by Odyssey SPAC, a total of 25.1 million ordinary shares with an agreed redemption price of €9.96 per share were redeemed for cash by eligible ordinary shareholders, following the redemption process. These are currently held as treasury shares. The redemption payable of €250.3 million was paid prior to Transaction close.
As part of the Capital Reorganisation, BenevolentAI Limited’s share capital was exchanged for shares in Odyssey Acquisition S.A. of £75k, being 90 million shares at a par value of €0.001. This capital reorganization reflects the transition of the share capital and share premium from BenevolentAI Ltd to BenevolentAI. This results in a decrease within share capital by £0.1m from the old share capital (par value of £0.10) with an increase of £584m reflecting a true-up to the premium on the shares issued to BenevolentAI shareholders in the share for share exchange. The corresponding adjustment to reflect the book value accounted, reflected through a corresponding charge to retained earnings of £43.2m and the merger difference of £535.9m, reflected in BenevolentAI.
The following table provides an overview of the impact of the Business Combination on the Statement of Comprehensive loss for the six months to 30 June 2022:
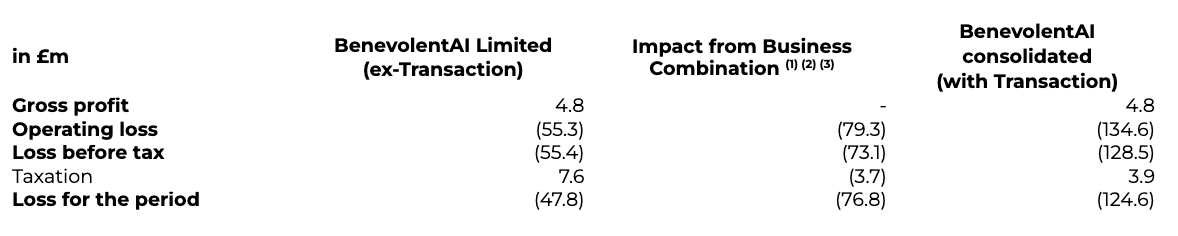
(1) Operating loss includes the listing service expense of £65.4m; Transaction-related SBP expenses of £2.6m; transaction costs of £11.3m; and costs incurred by the Company since Transaction close.(2) Loss before tax includes those adjustments noted above, in addition to the revaluation of warrants since Close of £6.1m credit.(3) Loss for the period includes those adjustments noted above, in addition to stamp duty tax payable of £3.7m.
In addition, the following table provides an overview of the impacts of the Business Combination on the Statement of Financial Position at 30 June 2022:
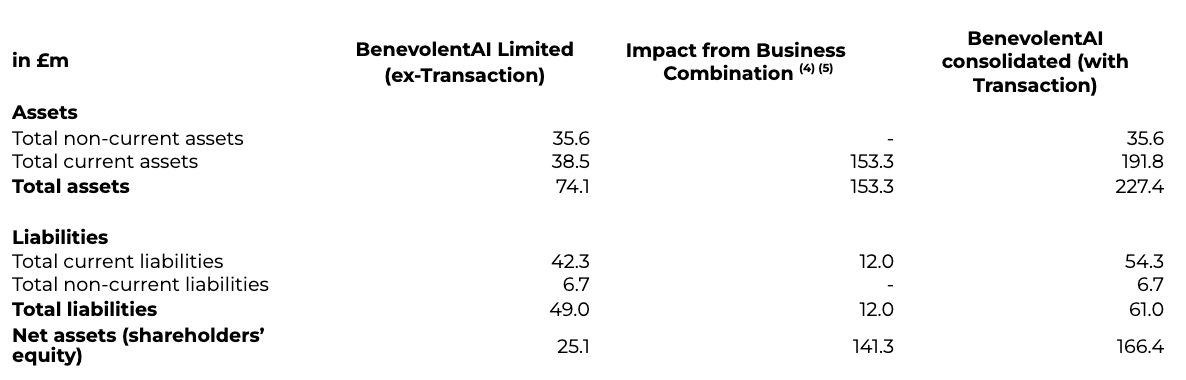
(4) Current assets include £32.0m proceeds from cash in Odyssey SPAC at Transaction close, in addition to £112.7m net proceeds from PIPE Financing and £33.2m proceeds from backstop facility. This is then net of outflows of £24.6m for payments of stamp duty; supplier invoices; and negative interest. Note, neither the PIPE Financing nor the backstop have been included as net assets when determining the listing service expense.(5) Current liabilities includes the £12.0m fair value of warrants as at 30 June 2022, having moved from the £18.1m valuation at the Transaction close.
5 Revenue
We initially recognise income under the AstraZeneca Collaboration as deferred revenue, which we become entitled to recognise as revenue in line with the delivery efforts towards the completion of tasks and provision of the deliverables set out in the agreements governing the AstraZeneca Collaboration. For the six months to 30 June 2022, this is represented by a revenue of £4.8m (six months to 30 June 2021: £1.7m).
AZ Collaboration deal
Building on the success of the first collaboration, the relationship with AstraZeneca has been expanded into a new 3-year partnership, starting 1 January 2022 and focusing on systemic lupus erythematosus (SLE) and heart failure (HF).
As the result of this collaboration BenevolentAI received an upfront fee of $15m (£11.8m) in January 2022. As the result of the upfront fee, a total of £7.0m deferred revenue is recognised as of 30 June 2022 (nil as at 31 Dec 2021).
There is no related party revenue in the six months to 30 June 2022 (six months to 30 June 2021: £nil). See note 15 for related party information.
6 Research and development expenditure
7 Finance income

8 Finance expense

9 Taxation

Research and Development Tax Credits represents estimated tax credits arising from the UK’s small and medium-sized enterprises R&D tax relief regime, which are settled in the following year. Receipts for 2021 remain outstanding due to a scheme wide backlog in processing, reviewing and settling submissions to claimants.
10 Loss per share
Loss per ordinary share has been calculated by dividing the loss attributable to equity holders of BenevolentAI after taxation for each financial period by the weighted average number of ordinary shares in issue during the financial period. The weighted average number of shares is calculated from the number of ordinary and preferred BenevolentAI shares in circulation at the beginning of the period adjusted by the number of ordinary shares issued during the period, alongside the impacts of the transaction and multiplied by a time-weighting factor. The time-weighting factor reflects the ratio of the number of days on which ordinary shares were issued and the total number of days of the period.
The G2 Growth Shares have been excluded as they do not attract dividends and were subsequently cancelled prior to the Transaction.
As the business combination is accounted for as if BenevolentAI Ltd has acquired Odyssey SPAC, the number of shares is adjusted to reflect the exchange ratio of the share for share exchange completed during the Transaction, such as to reflect the capital structure of the legal parent. In accordance with IAS 33, the calculation of the basic and diluted loss per share for all periods presented has been adjusted retrospectively due to these changes.
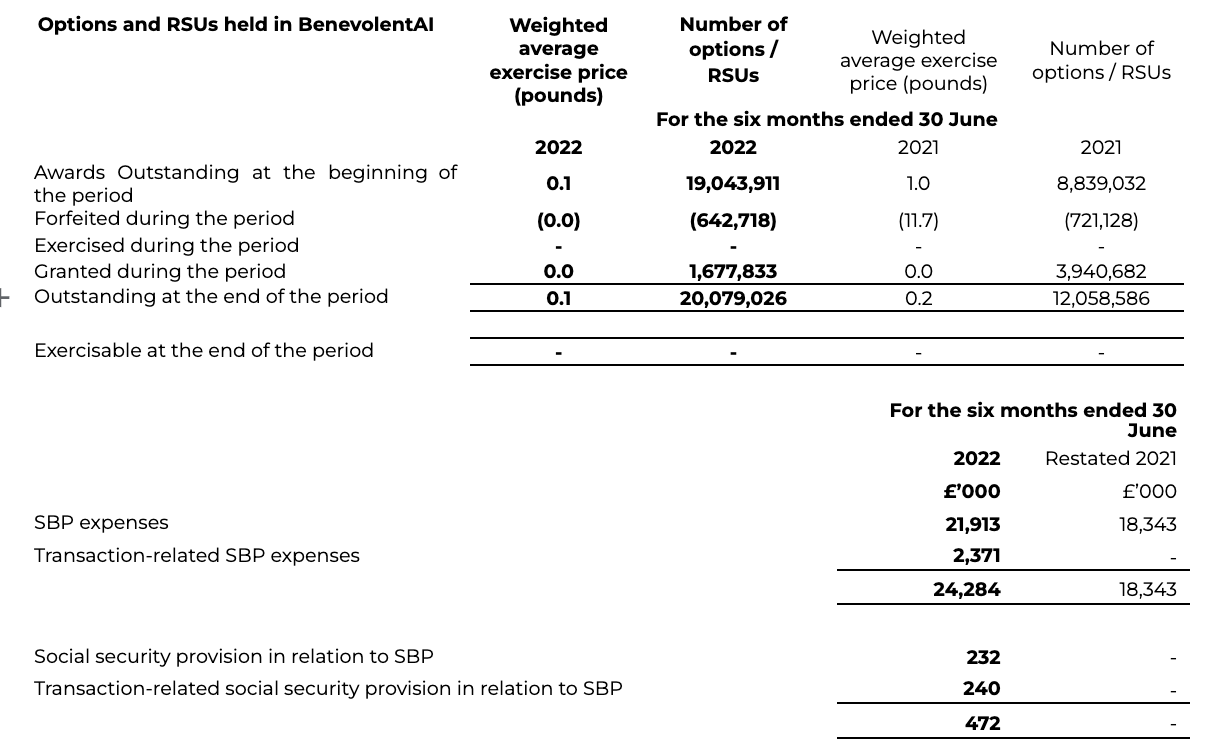
11 Share based payments (“SBP”)
During the period to the 22 April 2022 (before the Transaction), 1,602,040 options and 75,793 Restricted Stock Units (RSUs) were granted to employees and others under the unapproved Share Option Plan for BenevolentAI Limited. No awards were made between the Transaction and 30 June 2022. Benevolent options and RSUs have been adjusted based on the ratio of 1 BenevolentAI Limited ordinary and A preferred share into approximately 38.4930 BenevolentAI ordinary shares, as determined in the business combination agreement as part of the share for share exchange. Correspondingly, the exercise price has been divided by the same ratio, such that the fair value charge remains consistent, with no other impact arising from this scheme modification. During the six months to 30 June 2022 575,894 options and 66,824 RSUs were forfeited. The comparative information presented has been adjusted retrospectively for this conversion, where applicable.
The number and weighted average exercise prices of share options under BenevolentAI are as follows:
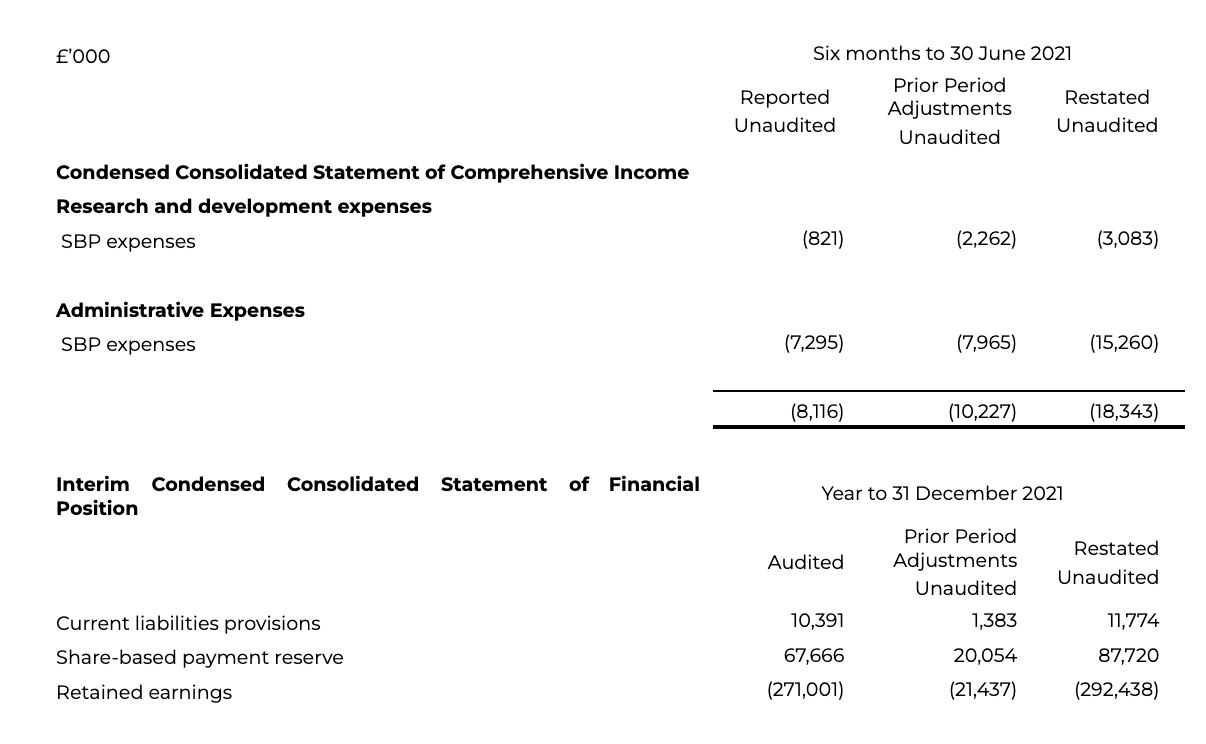
The Group has re-assessed the fair value (“FV”) charging methodology for the unapproved share option plan, identified as a result of the Business Combination, reflecting the scheme ‘point of exit’. Historic FV allocation was to spread straight line over the service period, rather than via a tranching approach. The tranching approach would result in straight line FV allocation for the tranches vesting pre-trigger over the ‘time to exit’, with the regular tranching thereafter. Prior estimates around the ‘time to exit’, using the service period as an estimate, were in some instances too long, meaning that the FV allocation in respect of awards was spread over a longer period than actually transpired. Indicators during 2021 would suggest that the re-assessment of the FV allocation over the period to exit should have taken place in 2021 and that the respective charges have been pulled back into the restated period.
The error and subsequent correction, presented through the restatement for the 2021 Interim Statement of Consolidated Income to 30 June and Full Year 2021 Statement of Financial Position is detailed below.
Audited balances referred to are pre-restatement, where the restatement itself is yet to be audited reflective of the interim statement itself being unaudited.
12 Trade and other Payables
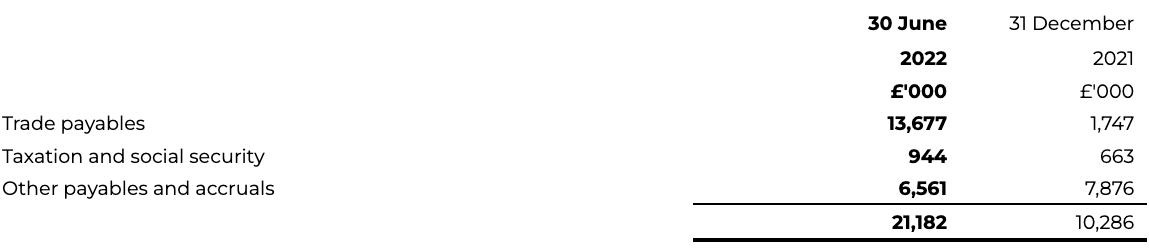
13 Warrants

The change in the fair value of the Warrants between 1 January 2022 to 21 April 2022 was recognised as retained earnings on the statement of the financial positions, prior to the Business Combination. Following the Transaction close and subsequent consolidation of the post-acquisition performance of BenevolentAI (formerly Odyssey SPAC), between 22 April 22 and 30 June 22, the change in the fair value was then recognised in the statement of comprehensive income as expense.
The expense for the warrants was determined on the basis of the fair value at the Closing date. The fair value was derived by applying the using Binomial Tree method and the Monte Carlo pricing method.
Monetary assets and liabilities denominated in foreign currencies at the balance sheet date are retranslated to the functional currency at the foreign exchange rate ruling at that date. Foreign exchange differences arising on translation are recognised in the Comprehensive income statement. The current warrants are EUR denominated liability and are retranslated as at 30 June 22. The Foreign exchange difference arising on the retranslation is posted as expense in the statement of comprehensive income.
14 Shareholdings
The table, including a comparative, reflects the shares in issue for BenevolentAI Limited as the accounting acquirer of Odyssey SPAC (now BenevolentAI) and the subsequent consolidated impact of the share for share exchanged, the impact of the redemption, equity Pipe Financing and equity Backstop facility for BenevolentAI, as the legal Parent and listed entity. Following the cancellation of the G2 Growth shares by BenevolentAI Limited, the Transaction involved the contribution of 2,338,423 existing BenevolentAI Limited shares held by BenevolentAI Limited shareholders against the issuance of new ordinary shares, adjusted based on the ratio of 1 BenevolentAI Limited share (Ordinary & A Preference) into approximately 38.4930 ordinary shares.
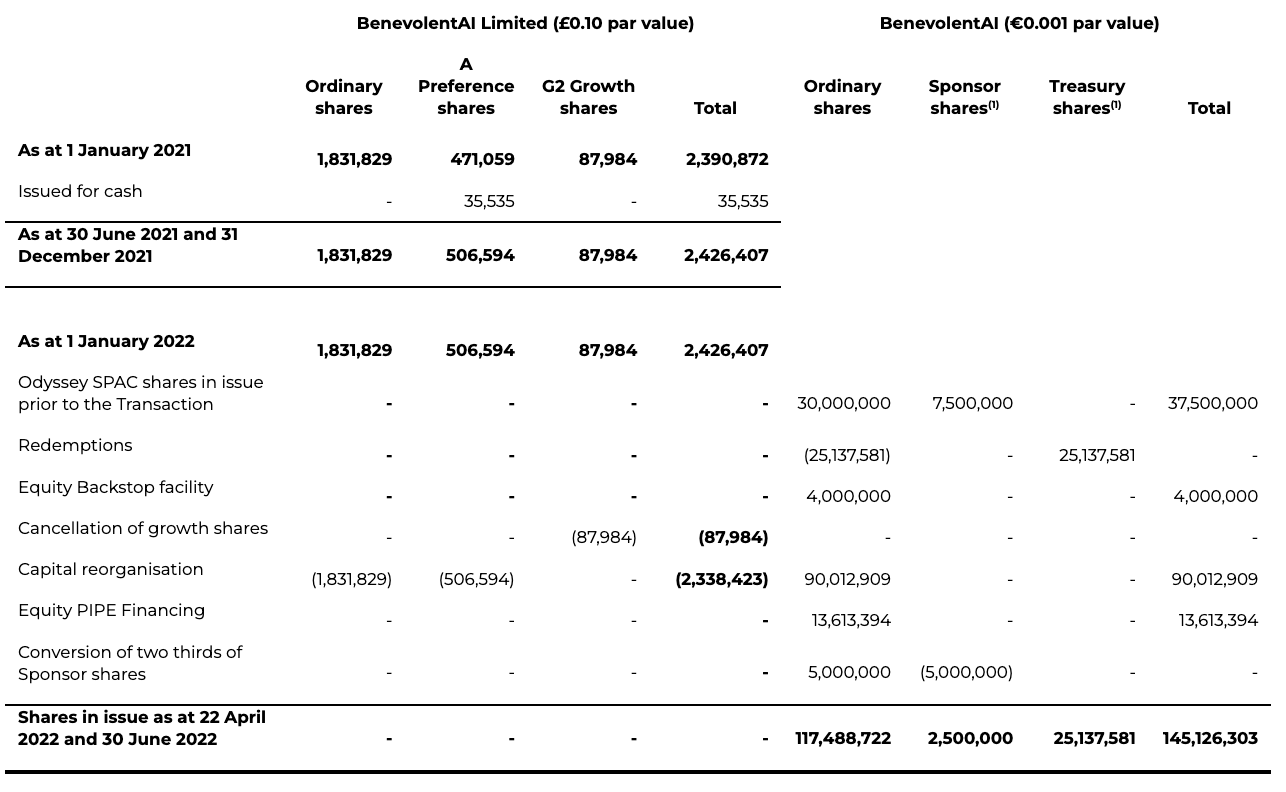
(1) The unconverted sponsor shares, and the treasury shares, do not form part of the Basic total number of ordinary shares outstanding. The sponsor shares derive their economic rights from their conversion to ordinary shares. The redemptions by ordinary shareholders ahead of the Closing date were transferred into treasury to be subsequently used to satisfy equity awards or be cancelled.
(2) The Capital reorganisation shows the impact of the share for share exchange on the BenevolentAI Ltd ordinary and preferred A shares in existence at closing, subject to an exchange ratio of 38.4930.
15 Related party transactions
There have been no material related party transactions in the first six months of 2022 and no material change in related parties from those described in the last annual report.
16 Subsequent events
There are no subsequent events to report.
17 Chief Executive Officer and Chief Financial Officer responsibility statement
We confirm, to the best of our knowledge, that:
1. The condensed consolidated financial statements of BenevolentAI for the period from 1 January 2022 to 30 June 2022 have been prepared in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board and as adopted by the European Union, and give a true and fair view of the assets, liabilities, financial position, profit or loss of Benevolent AI and the Group taken as a whole; and;
2. this report includes a fair review of the development and performance of the business and position of Benevolent AI and the Group taken as a whole for the first six months of the year, together with a description of the principal risks and uncertainties they face for the remaining six months of the year.
Joanna Shields, Chief Executive Officer
Nicholas Keher, Chief Financial Officer
Back to press releases and in the media



